Learning Objective
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Who will benefit from this module?
Manufacturing personnel in the pharma/biotech, dietary supplement, and medical devices industries need to understand the principles and practice of validation, as set out in this module. In particular, the module provides essential learning for engineering, production, and quality management personnel in the pharmaceutical industry.
Learning Objectives
- Describe the purpose and scope of validation master plans, outline their typical structure and contents, and explain their importance to management
- Contribute to the creation of project validation plans and protocols
- Identify important validation documents, specify their interrelationships, and describe how they are created and maintained
- Prepare and use validation schedules and resource plans, explain the basics of change control, and outline regulatory requirements for reporting and validating manufacturing changes
Module Outline
Validation master plan - This session describes the purpose and scope of validation master plans. It outlines the structure and contents of a typical validation master plan.
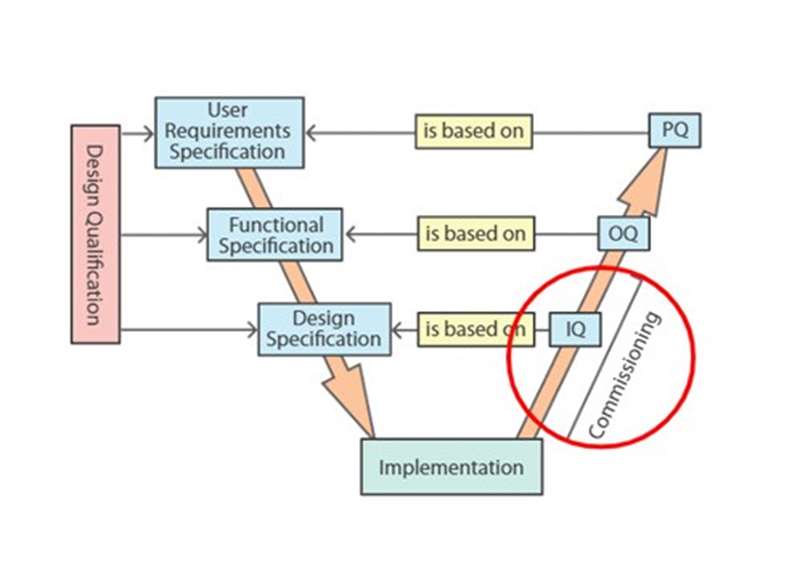
Project validation plan - This session describes how to use risk assessment to establish the scope of a project validation plan. It distinguishes prospective validation, continuous process verification, and concurrent validation. It identifies equipment and services that typically require qualification.
Validation documentation - This session identifies important validation documents and specifies their interrelationships. It outlines responsibilities and systems for control and approval of documentation in a validation project. It explains how to contribute to the development of validation protocols. It outlines how deviations and failures are dealt with, and the handling of raw data and reports. Finally, it describes procedures for tracking, cataloguing and archiving validation documents.
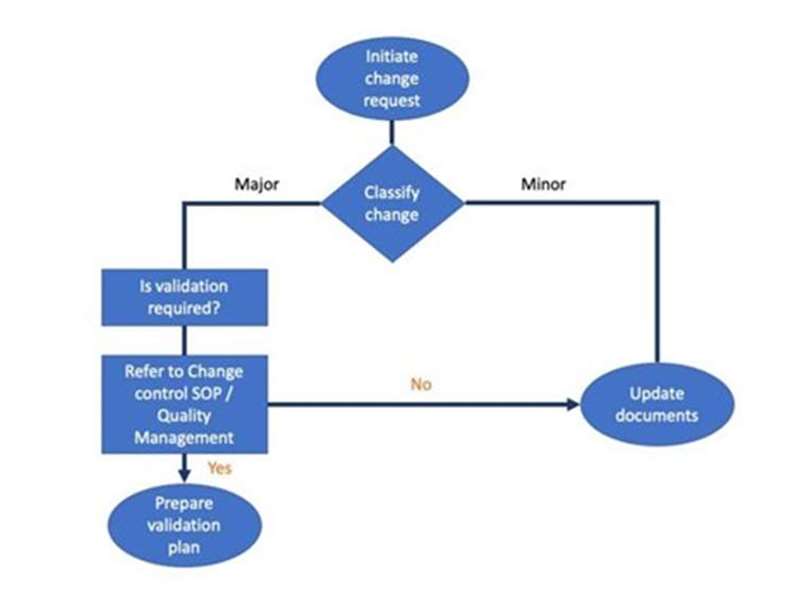
Scheduling, resource planning and change control - This session describes the purpose and use of validation schedules and validation resource plans. It discusses revalidation requirements in change management, and outlines requirements for reporting manufacturing changes to regulators.
Assessment - The assessment tests the learner’s assimilation of the module’s content.